New York, USA, Nov. 26, 2025 (GLOBE NEWSWIRE) -- Chemotherapy-induced Peripheral Neuropathy Treatment Market Poised for Strong Growth Throughout the Forecast Period (2025–2034) Amid Rising Chemotherapy Adoption | DelveInsight
The chemotherapy-induced peripheral neuropathy market is emerging as a high-value market opportunity driven by the lack of approved therapies and the significant unmet clinical need. Rising global chemotherapy usage, especially with neurotoxic agents, continues to expand the patient pool. Additionally, the launch of emerging therapies such as Halneuron (Wex Pharmaceuticals), ATX01 (AlgoTx), ART-123 (Asahi Kasei Pharma/Veloxis Pharmaceuticals), WST-057 (WinSanTor, Inc.), and others will further fuel the CIPN market growth.
DelveInsight’s Chemotherapy-induced Peripheral Neuropathy Market Insights report includes a comprehensive understanding of current treatment practices, emerging CIPN drugs, market share of individual therapies, and current and forecasted CIPN market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
Chemotherapy-induced Peripheral Neuropathy Market Summary
- The total CIPN treatment market size is expected to grow positively by 2034 in the leading markets.
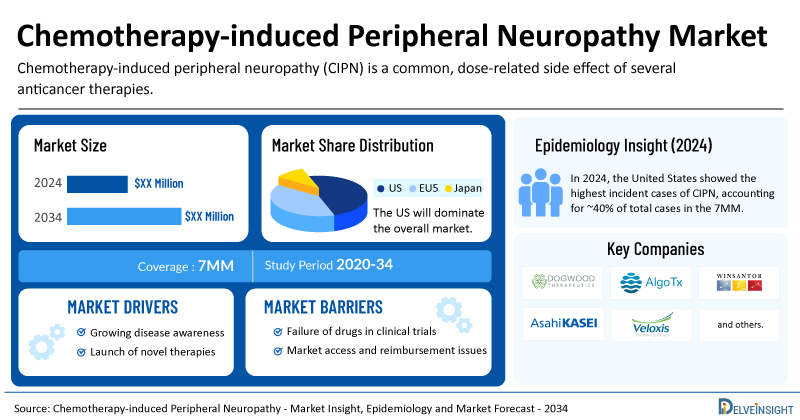
- The United States accounts for the largest market size of CIPN, in comparison to EU4 (Germany, Italy, France, and Spain), the UK, and Japan.
- In 2024, the United States showed the highest incident cases of CIPN, accounting for ~40% of total cases in the 7MM. These cases are expected to increase by 2034 by a decent CAGR.
- Key CIPN companies, including Dogwood Therapeutics, AlgoTx, Asahi Kasei Pharma, Veloxis Pharmaceuticals, WinSanTor, Inc., and others, are actively working on innovative CIPN drugs.
- Some of the key CIPN therapies in clinical trials include Halneuron (tetrodotoxin), ATX01, ART-123 (recombinant thrombomodulin alfa), WST-057, and others. These novel CIPN therapies are anticipated to enter the CIPN market in the forecast period and are expected to change the market.
Discover which CIPN medications are expected to grab the market share @ Chemotherapy-induced Peripheral Neuropathy Market Report
Key Factors Driving the Growth of the CIPN Market
Rising Cancer Incidence & Chemotherapy Usage
The increasing incidence of cancer worldwide, fueled by aging populations, changing lifestyles, and environmental factors, leads to greater use of chemotherapy treatments. As more patients survive cancer due to improved detection and therapy, prolonged and repeated chemotherapy raises the risk and awareness of CIPN.
Broader Adoption of Multimodal and Non-Pharmacological Therapies
Complementary approaches, such as acupuncture, transcutaneous electrical nerve stimulation (TENS), and other non-pharmacological interventions, are increasingly accepted as part of comprehensive care to relieve symptoms and improve quality of life.
Emergence of Novel CIPN Drugs
A few emerging therapies, such as Halneuron (Wex Pharmaceuticals), ATX01 (AlgoTx), ART-123 (Asahi Kasei Pharma/Veloxis Pharmaceuticals), WST-057 (WinSanTor, Inc.), and others, are in late-stage clinical development.
Chemotherapy-induced Peripheral Neuropathy Market Analysis
At present, no approved treatments exist specifically for CIPN. Management largely relies on off-label medications aimed at easing symptoms. Although numerous agents commonly used for neuropathic pain have been evaluated, none have shown clear effectiveness in preventing CIPN.
Patients experiencing CIPN symptoms are generally prescribed off-label drug classes such as alpha-2-delta ligands (anticonvulsants), serotonin–norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), opioids, and topical analgesics, all of which are standard options for neuropathic pain relief.
Several late-stage investigational therapies, including HALNEURON (tetrodotoxin/TTX), ATX01, and ART-123, are progressing through advanced clinical trials. The introduction of these new treatments between 2025 and 2034 is expected to reshape the CIPN landscape across the 7MM, potentially driving a shift toward disease-modifying therapeutic strategies and boosting market growth.
Learn more about the CIPN treatment options @ Chemotherapy-induced Peripheral Neuropathy Treatment Market
Chemotherapy-induced Peripheral Neuropathy Competitive Landscape
Some of the CIPN drugs in clinical trials include Halneuron (Wex Pharmaceuticals), ATX01 (AlgoTx), ART-123 (Asahi Kasei Pharma/Veloxis Pharmaceuticals), WST-057 (WinSanTor, Inc.), and others.
Wex Pharmaceuticals’ Halneuron (tetrodotoxin, TTX) is a small-molecule therapy aimed at treating moderate to severe neuropathic pain without the typical side effects of opioids. TTX works by blocking voltage-gated sodium channels (VGSCs) on pain-conducting nerves, which play a key role in chronic pain. The US FDA has granted Halneuron fast-track status for treating chemotherapy-induced neuropathic pain (CINP). It is currently in a Phase IIb trial enrolling patients with CIPN resulting from platinum- or taxane-based chemotherapy.
AlgoTx’s ATX01 is a first-in-class topical treatment for peripheral neuropathic pain, with a primary focus on CIPN. The formulation is designed to act locally within the epidermis and dermis, where nerve fiber damage occurs, thereby reducing systemic exposure and minimizing side effects. AlgoTx recently completed the ACT Phase II study in the US and Europe, assessing ATX01 in patients with CIPN.
Asahi Kasei Pharma/Veloxis Pharmaceuticals’ ART-123 is a recombinant human soluble thrombomodulin containing the extracellular region of thrombomodulin. It exerts anticoagulant activity by enhancing protein C activation and also displays anti-inflammatory and anti-fibrinolytic effects through activation of TAFI. Additional anti-inflammatory actions have been reported through direct binding to HMGB1 and promoting its thrombin-mediated degradation. ART-123 is currently in a Phase III clinical trial in the US for treating CIPN.
The anticipated launch of these emerging CIPN therapies are poised to transform the CIPN market landscape in the coming years. As these cutting-edge CIPN therapies continue to mature and gain regulatory approval, they are expected to reshape the CIPN market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for CIPN, visit @ Chemotherapy-induced Peripheral Neuropathy Medication
Recent Developments in the CIPN Market
- In August 2025, Dogwood Therapeutics announced that it had enrolled the first 50 patients in its ongoing Phase IIb CINP trial and remained on schedule to conduct an interim analysis of approximately 100 patients who are expected to complete the four-week study in the fourth quarter of this year.
- In June 2025, Asahi Kasei Pharma announced that administration of the study drug had begun for the first patient in a Phase III study of ART-123 in Japan for the prevention of sensory symptoms associated with CIPN.
- In February 2025, AlgoTherapeutix announced the completion of its 276 patient Phase II trial known as ‘ACT’ (ATX01 in CIPN).
What is Chemotherapy-induced Peripheral Neuropathy?
Chemotherapy-induced peripheral neuropathy (CIPN) is a common, dose-related side effect of several anticancer therapies—such as platinum compounds, taxanes, epothilones, vinca alkaloids, and newer drugs like bortezomib. It often forces clinicians to lower doses or stop treatment altogether, and significantly diminishes the quality of life for cancer survivors.
Chemotherapy-induced Peripheral Neuropathy Epidemiology Segmentation
The CIPN epidemiology section provides insights into the historical and current CIPN patient pool and forecasted trends for the leading markets. In 2024, the highest number of CIPN cases in the United States was observed in moderate CIPN with ~360K cases, followed by mild and severe cases.
The CIPN market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets segmented into:
- Total Incident Cases of CIPN
- Severity-specific Incident Cases of CIPN
- Incident Cases of CIPN by Chemotherapeutic Agents
- Incident Cases of CIPN by Cancer Type
- Total Treated Cases of CIPN
Download the report to understand CIPN management @ Chemotherapy-induced Peripheral Neuropathy Treatment Options
| Chemotherapy-induced Peripheral Neuropathy Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Chemotherapy-induced Peripheral Neuropathy Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Chemotherapy-induced Peripheral Neuropathy Epidemiology Segmentation | Total Incident Cases of CIPN, Severity-specific Incident Cases of CIPN, Incident Cases of CIPN by Chemotherapeutic Agents, Incident Cases of CIPN by Cancer Type, and Total Treated Cases of CIPN |
| Key Chemotherapy-induced Peripheral Neuropathy Companies | Dogwood Therapeutics, AlgoTx, Asahi Kasei Pharma, Veloxis Pharmaceuticals, WinSanTor, Inc., and others |
| Key Chemotherapy-induced Peripheral Neuropathy Therapies | Halneuron (tetrodotoxin), ATX01, ART-123 (recombinant thrombomodulin alfa), WST-057, and others |
Scope of the Chemotherapy-induced Peripheral Neuropathy Market Report
- Chemotherapy-induced Peripheral Neuropathy Therapeutic Assessment: Chemotherapy-induced Peripheral Neuropathy current marketed and emerging therapies
- Chemotherapy-induced Peripheral Neuropathy Market Dynamics: Conjoint Analysis of Emerging Chemotherapy-induced Peripheral Neuropathy Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Chemotherapy-induced Peripheral Neuropathy Market Unmet Needs, KOL’s views, Analyst’s views, Chemotherapy-induced Peripheral Neuropathy Market Access and Reimbursement
Discover more about CIPN drugs in development @ Chemotherapy-induced Peripheral Neuropathy Clinical Trials
Table of Contents
| 1 | Chemotherapy-induced Peripheral Neuropathy Market Key Insights |
| 2 | Report Introduction |
| 3 | Chemotherapy-induced Peripheral Neuropathy Market Overview at a Glance |
| 3.1 | Market Share (%) Distribution of Chemotherapy-induced Peripheral Neuropathy (CIPN) in 2020 |
| 3.2 | Market Share (%) Distribution of Chemotherapy-induced Peripheral Neuropathy (CIPN) in 2034 |
| 4 | Methodology of Epidemiology and Market |
| 5 | Executive Summary of Chemotherapy-induced peripheral neuropathy (CIPN) |
| 6 | Key Events |
| 7 | Disease Background and Overview |
| 7.1 | Introduction |
| 7.2 | Clinical Features |
| 7.3 | Symptoms of CIPN |
| 7.4 | Grading of chemotherapy-induced peripheral neuropathy |
| 7.5 | Pathophysiology of CIPN |
| 7.6 | Chemotherapy-induced Peripheral Neuropathy: Clinical Presentation |
| 7.7 | Genetics of chemotherapy-induced peripheral neuropathy |
| 7.9 | Treatment and Management of CIPN |
| 8 | Epidemiology and Patient Population |
| 8.1 | Key Findings |
| 8.2 | Assumptions and Rationale: The 7MM |
| 8.2.1 | Total Incident Cases of CIPN |
| 8.2.2 | Severity-specific Incident Cases of CIPN |
| 8.2.3 | Incident Cases of CIPN by Chemotherapeutic Agents |
| 8.2.4 | Incident Cases of CIPN by Cancer Type |
| 8.2.5 | Total Treated Cases of CIPN |
| 8.3 | Total Incident Cases of CIPN in 7MM |
| 8.4 | The United States |
| 8.4.1 | Total Incident Cases of CIPN in the United States |
| 8.4.2 | Severity-specific Incident Cases of CIPN in the United States |
| 8.4.3 | Incident Cases of CIPN by Chemotherapeutic Agents in the United States |
| 8.4.4 | Incident Cases of CIPN by Cancer Type in the United States |
| 8.4.5 | Total Treated Cases of CIPN in the United States |
| 8.5 | EU4 and the UK |
| 8.6 | Japan |
| 9 | Patient Journey |
| 10 | Emerging CIPN Therapies |
| 10.1 | Key Cross Competition |
| 10.2 | Halneuron (Tetrodotoxin): Dogwood Therapeutics |
| 10.2.1 | Product Description |
| 10.2.2 | Other Development Activities |
| 10.2.3 | Clinical Trial Information |
| 10.2.4 | Safety and Efficacy |
| 10.2.5 | Analysts' Views |
| 10.3 | ATX01: AlgoTx |
| List of drugs to be continued in the report... | |
| 11 | CIPN Market: Market Analysis |
| 11.1 | Key Findings |
| 11.2 | Key CIPN Market Forecast Assumptions |
| 11.3 | CIPN Market Outlook |
| 11.4 | Conjoint Analysis |
| 11.5 | Total Market Size of CIPN in the 7MM |
| 11.6 | Market size of CIPN by Therapies in the 7MM |
| 11.7 | Market Size of CIPN in the United States |
| 11.7.1 | Total Market Size of CIPN in the United States |
| 11.7.2 | Market Size by Therapies of CIPN in the United States |
| 11.8 | Market Size of CIPN in EU4 and the UK |
| 11.9 | Market Size of CIPN in Japan |
| 12 | KOL Views on CIPN |
| 13 | CIPN Market SWOT Analysis |
| 14 | CIPN Market Unmet Needs |
| 15 | CIPN Market Access and Reimbursement |
| 15.1 | The United States |
| 15.2 | In EU4 and the UK |
| 15.3 | Japan |
| 16 | Bibliography |
| 17 | Acronyms and Abbreviations |
| 18 | CIPN Report Methodology |
Related Reports
Chemotherapy-induced Peripheral Neuropathy Clinical Trial Analysis
Chemotherapy-induced Peripheral Neuropathy Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key CIPN companies, including Grünenthal GmbH, AlgoTherapeutix, Wex Pharmaceuticals Inc., among others.
Peripheral Neuropathic Pain Market
Peripheral Neuropathic Pain Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key peripheral neuropathic pain companies, including Lexicon Pharmaceuticals, Merz Therapeutics, Apurano Pharmaceuticals, Vertex Pharmaceuticals, WEX Pharmaceuticals, Scilex Holding Company, among others.
Peripheral Neuropathic Pain Clinical Trials Analysis
Peripheral Neuropathic Pain Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Peripheral Neuropathic Pain companies, including Haisco Pharmaceutical Group, GlaxoSmithKline, Algiax Pharmaceuticals, Apurano Pharmaceuticals GmbH, Eli Lilly and Company, Shanghai SIMR Biotechnology, Lexicon Pharmaceuticals, among others.
Diabetic Peripheral Neuropathy Market
Diabetic Peripheral Neuropathy Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key diabetic peripheral neuropathy companies including Grünenthal, Averitas Pharma, Daiichi Sankyo, Helixmith, Vertex Pharmaceuticals, among others.
Diabetic Neuropathy Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key diabetic neuropathy companies, including Novo Nordisk, Ionis Pharmaceuticals, Pfizer, Regeneron, NeuroMetrix, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
